Are you ready to find 'write a balanced half reaction for the oxidation of water'? You can find questions and answers on the topic here.
Body of water oxidation is ane of the fractional reactions of body of water splitting : 2H 2 O → O 2 + 4H + + 4e − Oxidization (generation of dioxygen) 4H + + 4e − → 2H 2 Diminution (generation of dihydrogen)
Table of contents
- Write a balanced half reaction for the oxidation of water in 2021
- Io2 to io3 half-reaction
- Writing and balancing complex half-reactions in basic solution
- Write a balanced half-reaction for the oxidation of hydrogen peroxide
- What is the half-reaction for the oxidation of mn metal
- Ash3 h3aso4 half-reaction
- Balanced half-reaction n2 to n2h4
- Write a balanced half-reaction for the reduction of permanganate ion to solid manganese dioxide
Write a balanced half reaction for the oxidation of water in 2021
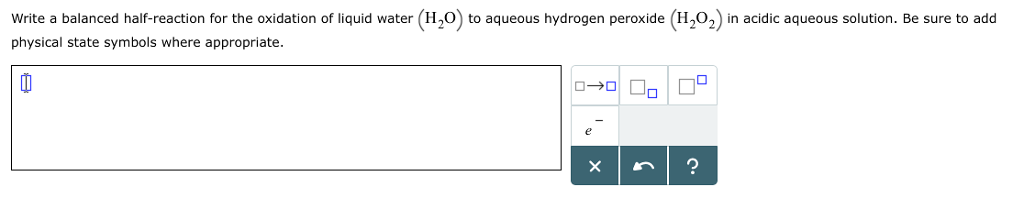 This picture shows write a balanced half reaction for the oxidation of water.
This picture shows write a balanced half reaction for the oxidation of water.
Io2 to io3 half-reaction
 This picture shows Io2 to io3 half-reaction.
This picture shows Io2 to io3 half-reaction.
Writing and balancing complex half-reactions in basic solution
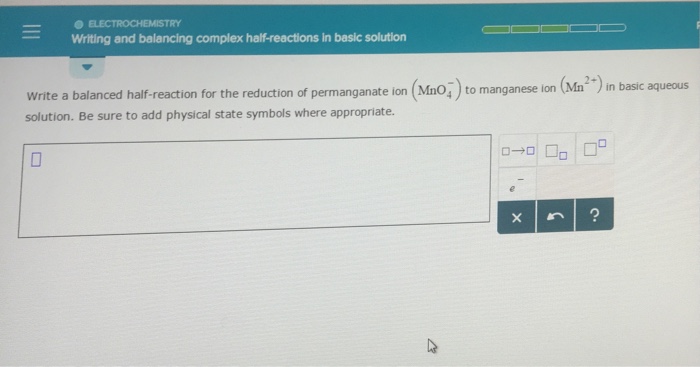 This image demonstrates Writing and balancing complex half-reactions in basic solution.
This image demonstrates Writing and balancing complex half-reactions in basic solution.
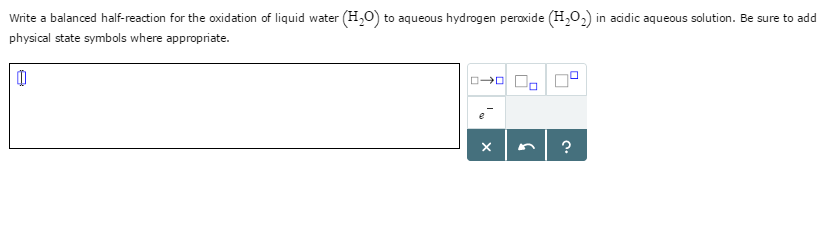
Write a balanced half-reaction for the oxidation of hydrogen peroxide
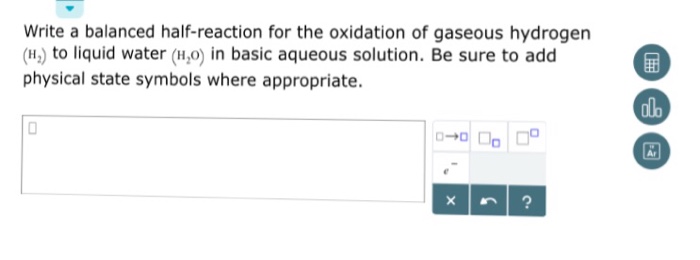 This picture shows Write a balanced half-reaction for the oxidation of hydrogen peroxide.
This picture shows Write a balanced half-reaction for the oxidation of hydrogen peroxide.
What is the half-reaction for the oxidation of mn metal
 This picture illustrates What is the half-reaction for the oxidation of mn metal.
This picture illustrates What is the half-reaction for the oxidation of mn metal.
Ash3 h3aso4 half-reaction
 This picture illustrates Ash3 h3aso4 half-reaction.
This picture illustrates Ash3 h3aso4 half-reaction.
Balanced half-reaction n2 to n2h4
 This image illustrates Balanced half-reaction n2 to n2h4.
This image illustrates Balanced half-reaction n2 to n2h4.
Write a balanced half-reaction for the reduction of permanganate ion to solid manganese dioxide
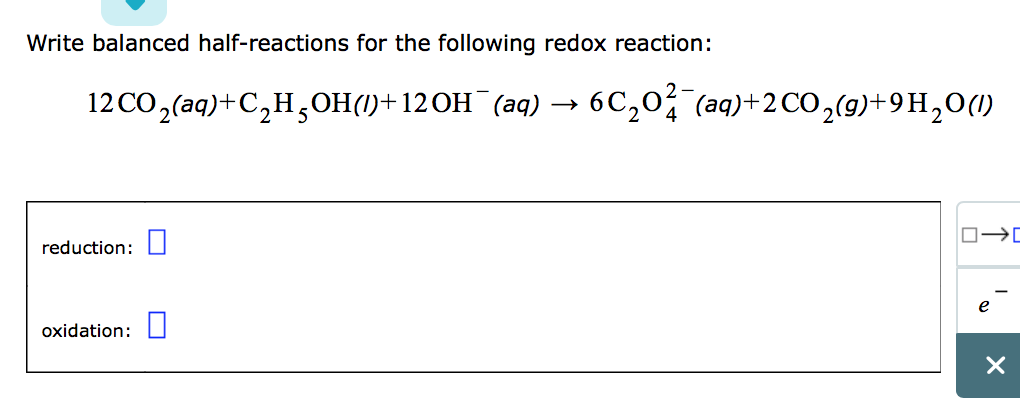 This image demonstrates Write a balanced half-reaction for the reduction of permanganate ion to solid manganese dioxide.
This image demonstrates Write a balanced half-reaction for the reduction of permanganate ion to solid manganese dioxide.
How to calculate the balance of an oxidation reaction?
Adding a single electron on the right side gives a balanced oxidation half-reaction: oxidation (balanced): Fe2+(aq)⟶ Fe3+(aq)+e− oxidation (balanced): Fe 2 + ( a q) ⟶ Fe 3 + ( a q) + e − You should check the half-reaction for the number of each atom type and the total charge on each side of the equation.
Which is the half reaction where water loses electrons?
In short, choose the one with the same charged particle as is used in the other half-reaction to make your life simpler. You are looking for a reaction where water is oxidized, i.e. loses electrons. In the chemical equation, the electrons must appear on the opposite side as H X 2 O, since electrons are products of an oxidation half-reaction.
What do you look for in an oxidation half reaction?
You are looking for a reaction where water is oxidized, i.e. loses electrons. In the chemical equation, the electrons must appear on the opposite side as HX2O, since electrons are products of an oxidation half-reaction. On the other hand, any time we write X + eX − we are depicting a reduction of X.
Which is the key to combining oxidation and reduction?
The key to combining the half-reactions is the electrons. The electrons lost during oxidation must go somewhere. These electrons go to cause reduction. The number of electrons transferred from the oxidation half-reaction to the reduction half-reaction must be equal.
Last Update: Oct 2021