Do you want to find 'write a balanced equation for the dissociation of calcium hydroxide'? You can find all the information on this website.
Table of contents
- Write a balanced equation for the dissociation of calcium hydroxide in 2021
- Ca(oh)2 molar mass
- Calcium hydroxide + water equation
- Write out the equilibrium equation for the dissolution of calcium hydroxide in water
- Ca(oh)2 dissociation in water
- Ca oh 2
- Dissociation of calcium hydroxide equation
- Calcium hydroxide equation
Write a balanced equation for the dissociation of calcium hydroxide in 2021
 This picture illustrates write a balanced equation for the dissociation of calcium hydroxide.
This picture illustrates write a balanced equation for the dissociation of calcium hydroxide.
Ca(oh)2 molar mass
 This picture demonstrates Ca(oh)2 molar mass.
This picture demonstrates Ca(oh)2 molar mass.
Calcium hydroxide + water equation
 This picture illustrates Calcium hydroxide + water equation.
This picture illustrates Calcium hydroxide + water equation.
Write out the equilibrium equation for the dissolution of calcium hydroxide in water
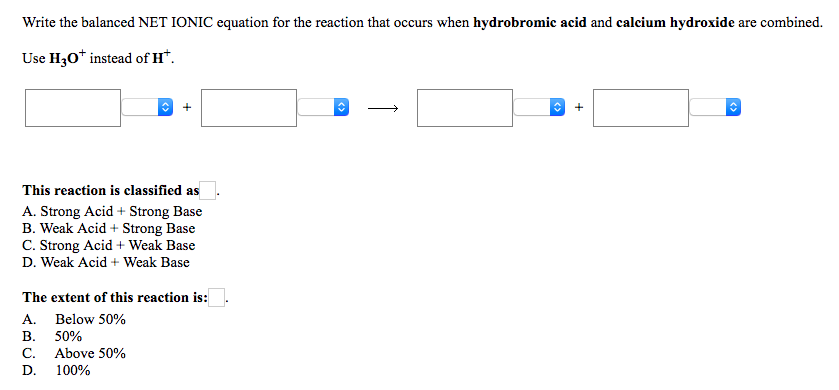 This picture demonstrates Write out the equilibrium equation for the dissolution of calcium hydroxide in water.
This picture demonstrates Write out the equilibrium equation for the dissolution of calcium hydroxide in water.
Ca(oh)2 dissociation in water
 This image illustrates Ca(oh)2 dissociation in water.
This image illustrates Ca(oh)2 dissociation in water.
Ca oh 2
 This picture representes Ca oh 2.
This picture representes Ca oh 2.
Dissociation of calcium hydroxide equation
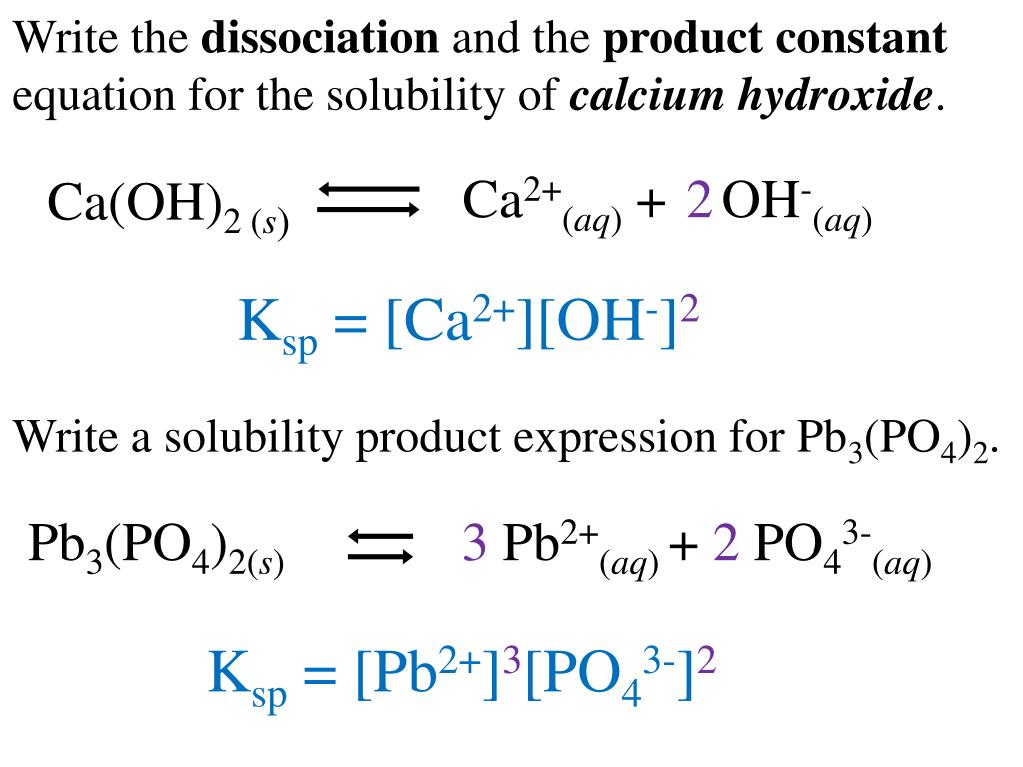 This picture demonstrates Dissociation of calcium hydroxide equation.
This picture demonstrates Dissociation of calcium hydroxide equation.
Calcium hydroxide equation
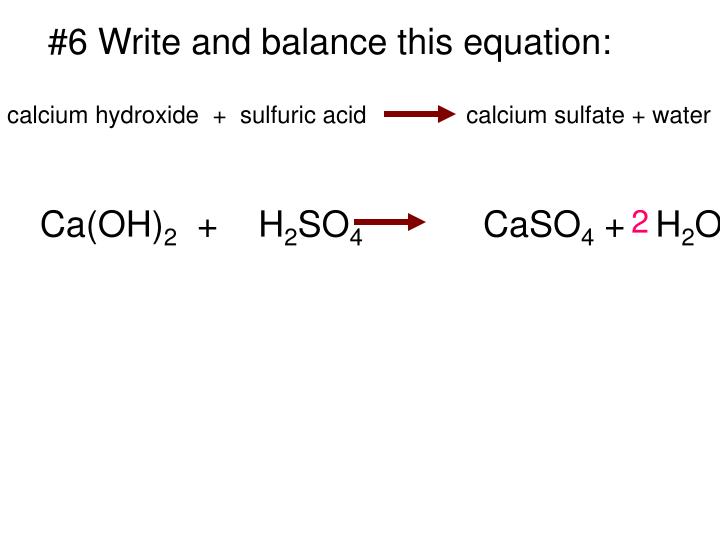 This image demonstrates Calcium hydroxide equation.
This image demonstrates Calcium hydroxide equation.
How do you write dissociation equations in chemistry?
Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. It is important to be able to write dissociation equations. Simply undo the crisscross method that you learned when writing chemical formulas of ionic compounds. The subscripts for the ions in the chemical formulas become the coefficients...
How to write the chemical equation for calcium hydroxide?
Write the chemical equation for the dissolution of calcium hydroxide in water, and write its corresponding K_sp equilibrium expression. Write the balanced chemical equation for the reaction of HCl with calcium hydroxide, which yields water and calcium chloride.
How to calculate the K sp of calcium hydroxide?
Calculating the K sp of Calcium Hydroxide. Introduction. Calcium hydroxide, Ca(OH) 2, is an ionic solid that is slightly soluble in water. A saturated solution is an equilibrium, that can be represented by the following equation: Ca(OH) 2 (s) Ca 2+ (aq) + 2OH - (aq)
What are the dissociation equations for NaCl and NH 4?
Shown below are dissociation equations for NaCl, Ca (NO 3) 2 , and (NH 4) 3 PO 4. The formula unit of sodium chloride dissociates into one sodium ion and one chloride ion. The calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions.
Last Update: Oct 2021